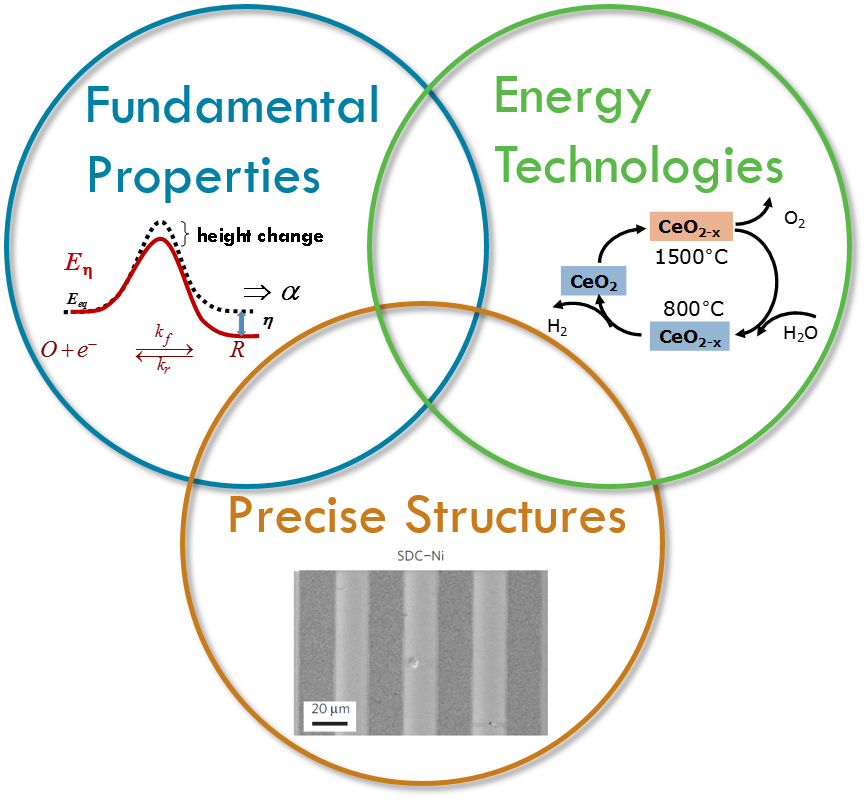
Overview
The work in Prof. Sossina Haile's research group broadly encompasses solid state ionic materials and devices, with a particular focus on energy technologies. Her group has worked to establish a new class of fuel cells based on solid acid elctrolytes and has demonstrated record power densities for solid oxide fuel cells. The group's more recent work on water and carbon dioxide dissociation for solar-fuel generation by thermochemical processes has created new avenues for harnessing sunlight to meet energy demands.
Specific materials under investigation in Dr. Haile's group include proton-conducting solid acid and perovskite electrolytes, mixed oxygen- and electron-conducting fluorites and perovskites, and non-stoichiometric oxides. Characterization tools range from A.C. impedance spectroscopy in a variety of configurations to x-ray and neutron scattering and thermal analysis. In many cases, specialized in-house tools are constructed allowing acquisition of data otherwise inaccessible using commercial instrumentation. Using these methods, her group has shown, for example, that defect trapping plays a significant role in limiting the magnitude of proton conductivity in yttrium-doped barium zirconate, one of the most promising electrolytes for reduced-temperature solid-oxide fuel cells.
Solar-driven Thermochemical Fuel Production
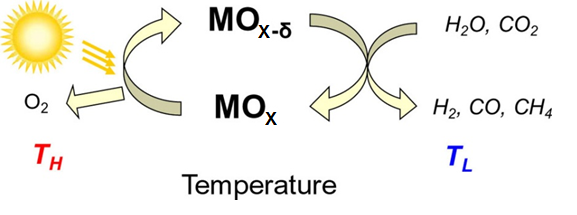
Variable valence oxides have the important characteristic that at high temperatures thermodynamics drives oxygen release, whereas at lower temperatures oxygen reabsorbtion is thermodynamically favored. At the lower temperature, if gases such as water or carbon dioxide are used to reoxidize the material, the reaction generates a reduced fuel such as hydrogen or carbon monoxide as the reaction product. Such thermochemical production, when driven by solar thermal heating, can be used for solar fuel generation. In this work we develop new materials with desirable thermodynamic and kinetic properties for thermochemical solar fuel synthesis. Our initial studies have focused on doped ceria and the results suggest that solar to fuel conversion efficiencies of ~20% can be achieved even in the absence of heat recovery and that fuels ranging from hydrogen and carbon monoxide to syngas and methane can be produced with extremely rapid kinetics. Our most recent work in this area has determined that at the highest temperatures (>1100°C) fuel production rates are limited by thermodynamics rather than material kinetics.
Proton Conducting Solid Acid Compounds
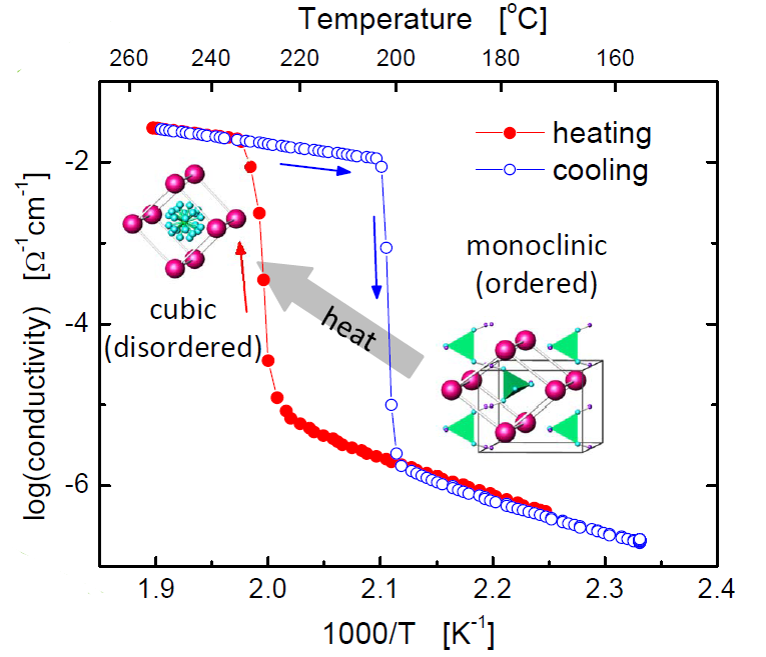
Solid acids are a fascinating class of materials built upon hydrogen bonded oxyanion groups. In contrast to polymeric proton conductors, these compounds conduct protons without the assistance of mobile water molecules, opening new technological possibilities and scientific avenues. Our success in fabricating and demonstrating fuel cells based on these electrolytes has led to the spin-off company SAFCell. The group's current research efforts focus on increasing platinum utilization through the use of carbon nanomaterials and electrospray deposition to increase electrode surface area.
You can find more information about these exciting materials via the Caltech publication ENGenious and from this video from the Science and Technology News Network.
Solid Oxide Fuel Cells
Solid oxide fuel cells (SOFCs) operate at high temperatures, and, as a consequence, they can utilize hydrocarbon fuels, they provide for efficient catalysis, and they exhaust 'high quality' waste heat that can be used for additional power generation. High temperature operation, however, has generally been responsible for the high cost of SOFCs and for their undesirability for portable applications. Our efforts in solid oxide fuel cells span a number of activities focused on reducing the operating temperature of SOFCs to "intermediate" temperatures (below ~600°C) where inexpensive stainless steel components can be used. Currently, these include high-throughput materials discovery of cathodes for high activity oxygen electroreduction, facilitating commercialization of SOFCs based on proton-conducting electrolytes, and investigating the potential for ceria as both an electrolyte and active anode material.